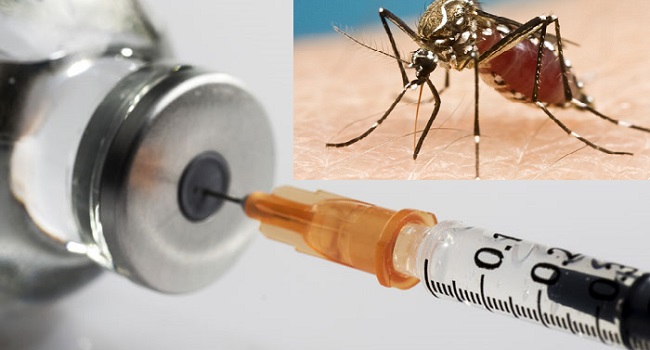
The World Health Organization approved the RTS,S/AS01 malaria vaccine on Wednesday, making it the first vaccination against the mosquito-borne disease that kills over 400,000 people each year, largely African children.
According to AFP, the decision was taken after an evaluation of a pilot program that has been running in Ghana, Kenya, and Malawi since 2019 and has handed out over two million doses of the vaccine, which was first developed by the pharmaceutical company GSK in 1987.
The WHO announced it was “recommending the widespread use of the world’s first malaria vaccine” after analyzing evidence from those nations, according to Tedros Adhanom Ghebreyesus, the agency’s director general.
Children in Sub-Saharan Africa and other regions with moderate to high malaria transmission should receive four doses up to the age of two, according to the WHO.
According to WHO estimates from 2019, more than half of malaria deaths occur in six Sub-Saharan African countries, with Nigeria accounting for over a quarter of all deaths.
Fever, headaches, and muscle discomfort are common symptoms, followed by chills, fever, and sweating in cycles.
According to Kate O’Brien, Director of WHO’s Department of Immunization, Vaccines, and Biologicals, the vaccine “substantially reduces severe malaria, which is the lethal variety, by 30%.”
The WHO regional director for Africa, Matshidiso Moeti, said Wednesday’s recommendation “offers a glimmer of hope for the continent which shoulders the heaviest burden of the disease.”
The agency hopes this latest recommendation will encourage scientists to develop more malaria vaccines.
Pedro Alonso, Director of the WHO Global Malaria Programme said the RTS,S/AS01 is “a first generation, really important one, but we hope… it stimulates the field to look for other types of vaccines to completement or go beyond this one.”